05 Dec Phosphorus based optoelectronics
Polycyclic aromatic hydrocarbons (PAHs) play a central role in a variety of electronic and optoelectronic applications, including chemical sensors, organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs) and organic solar cells. In order to optimize the performance of the components and increase their versatility, researchers are testing substitution with various elements beyond carbon. While substitution with boron (B), nitrogen (N), oxygen (O) and sulphur (S) has already been extensively researched, the integration of phosphorus (P) in combination with nitrogen (N) still poses a major challenge.
New compounds for organic semiconductors
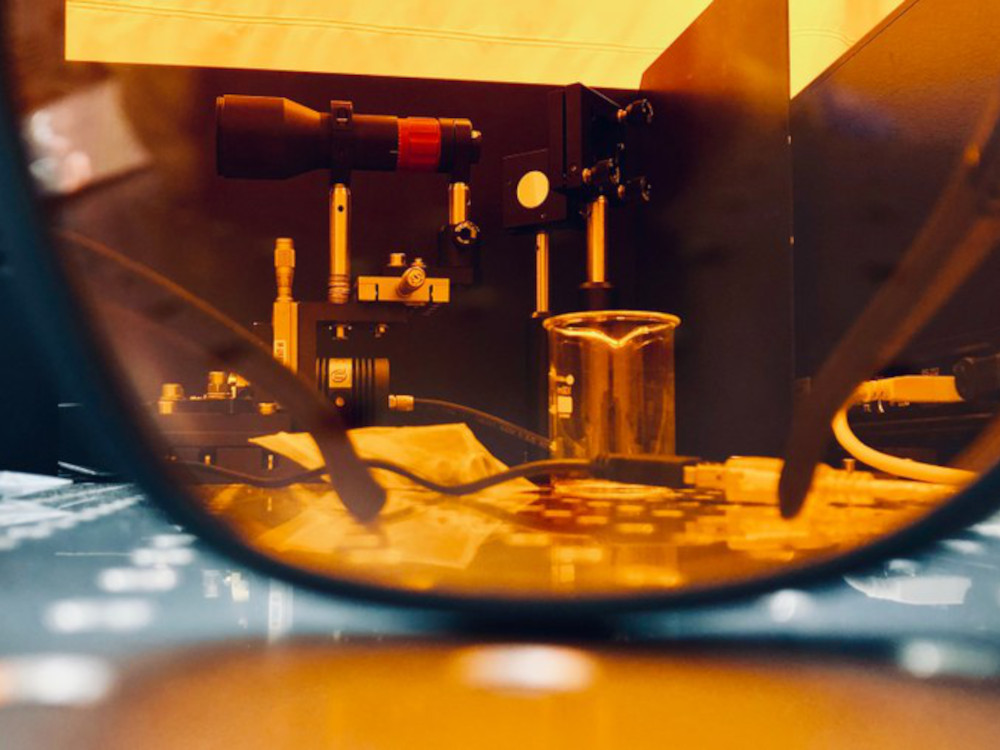
In collaboration with an interdisciplinary team, phosphorus chemist Professor Jan. J. Weigand from the Technische Universität Dresden has now developed a new method for introducing phosphorus and nitrogen atoms into polycyclic molecules. “This method made it possible to synthesize a wide range of P/N-substituted compounds, whose physicochemical properties were investigated in many different ways in collaboration with physicists at TU Dresden,” explains Weigand. “By combining material simulations and spectroscopic measurements, we were able to gain fundamental insights into the structure-property relationships of the resulting compounds.” In future, the method could enable the development of new materials with specific optoelectronic properties for use in organic semiconductor technologies such as OLEDs or sensors.
Polycyclic molecules with phosphorus and nitrogen should open up new possibilities for electronic and optoeletronic components. Image: Jannis Fidelius
The new approach makes the well-known class of azaphospholes accessible, which was previously very difficult to access and usually in very low yields. It has therefore not been considered for (opto)electronic applications until now. “Through the targeted combination of phosphorus and nitrogen, we hope to be able to control the electronic and optical properties of these compounds in a way that was previously not possible,” adds Sebastian Reineke, head of the Light-Emitting and Excitonic Organic Semiconductors (LEXOS) group at TU Dresden. This opens up exciting prospects for future applications in optoelectronics and beyond”
Original publication:
[Jannis Fidelius, et al, Convenient Access to π-Conjugated 1,3-azaphospholes from Alkynes via [3+2]-Cycloaddition and Reductive Aromatization, CHEM. DOI: 10.1016/j.chempr.2023.10.016]
Source: tu-dresden.de
Image: Sebastian Reineke